Mario Mikula, Assoc. Prof. Priv. Doz. Mag. Dr., MAS
Genetiker und ToxikologeTel: + 43 (0)1 40160-56540
E-Mail: mario.mikula@meduniwien.ac.at
Research Focus
Differentiation programs active during melanoma development
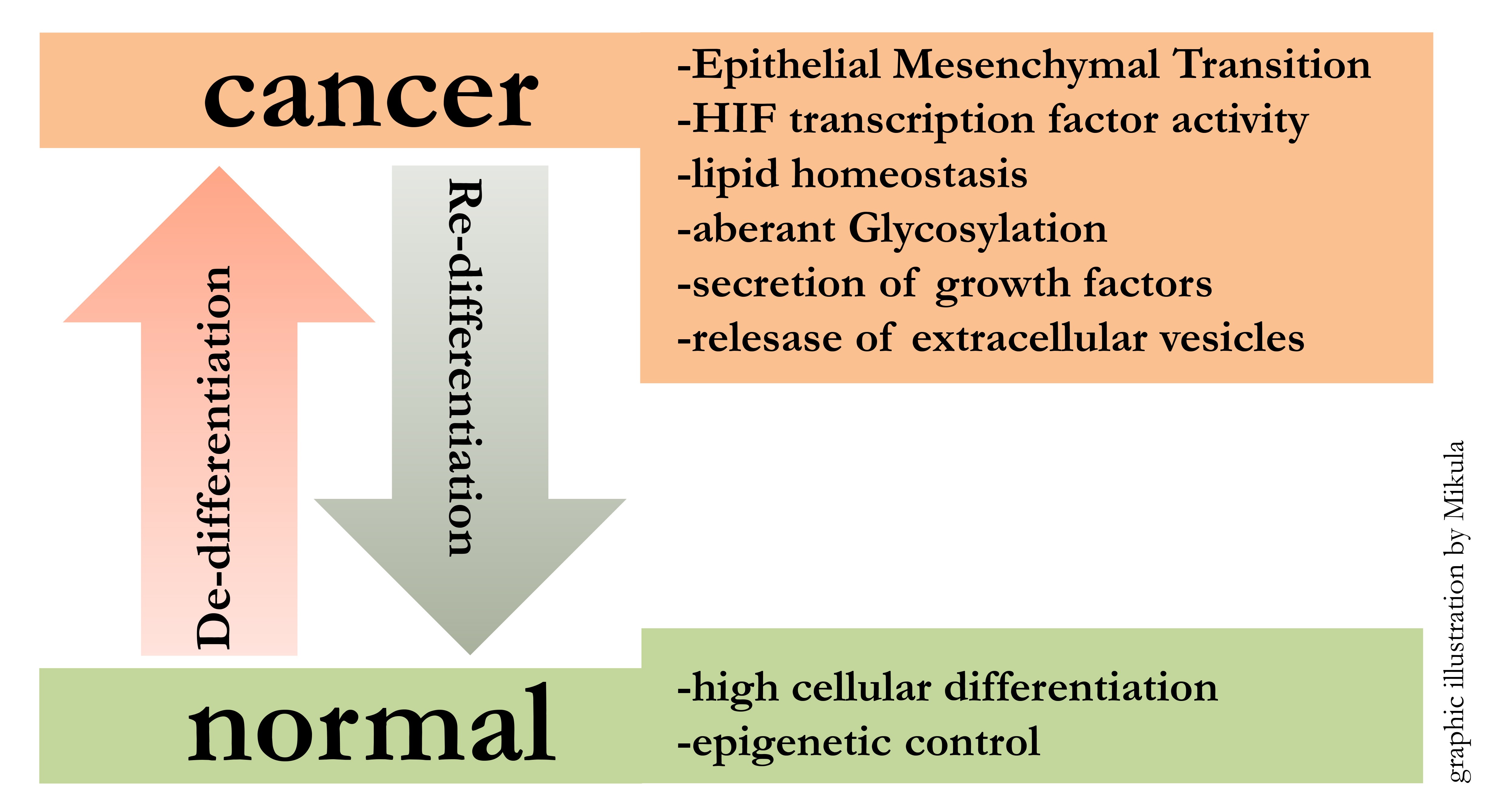
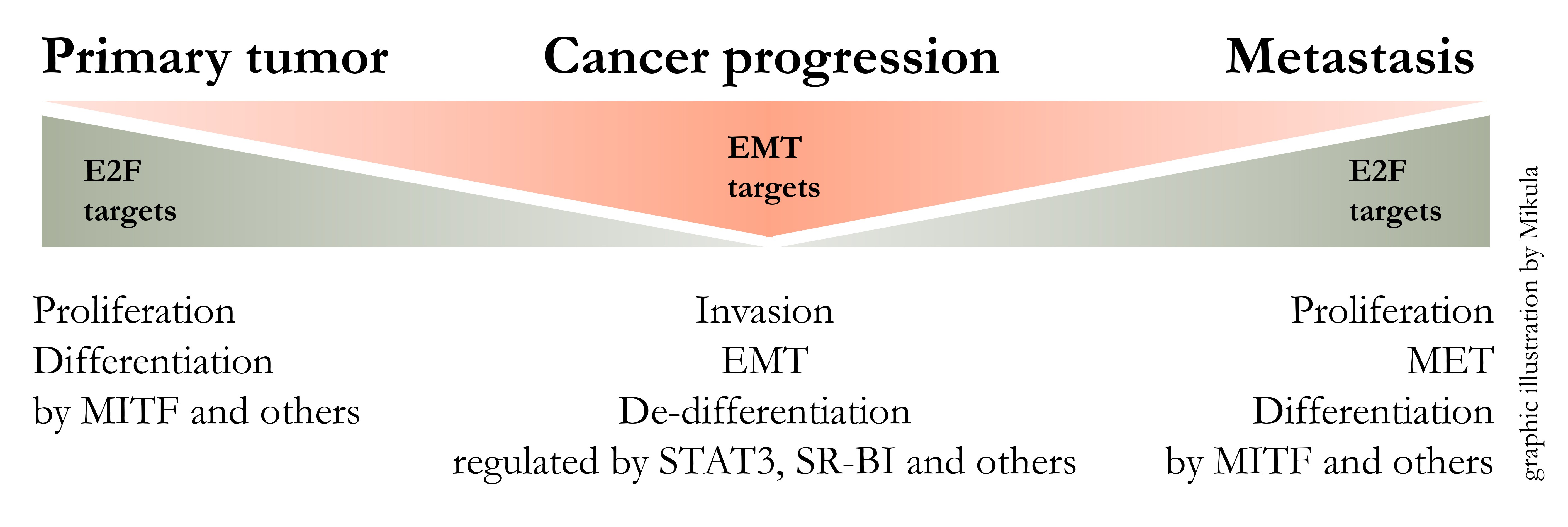
Starting from embryonic stem cells, developmental programs lead to the formation of all organs in the human body. This implies that cellular differentiation mechanisms are powerful determinators of cellular fate. The reactivation of these conserved developmental programs, like EMT (Epithelial Mesenchymal Transition), during and after pathologic events, has profound impact on the outcome of disease. Focussing on cancer, de-differentiation along with tumor progression can be observed. In particular our research has shown that melanoma cells loose differentiation and at the same time gain cancer specific characteristics.
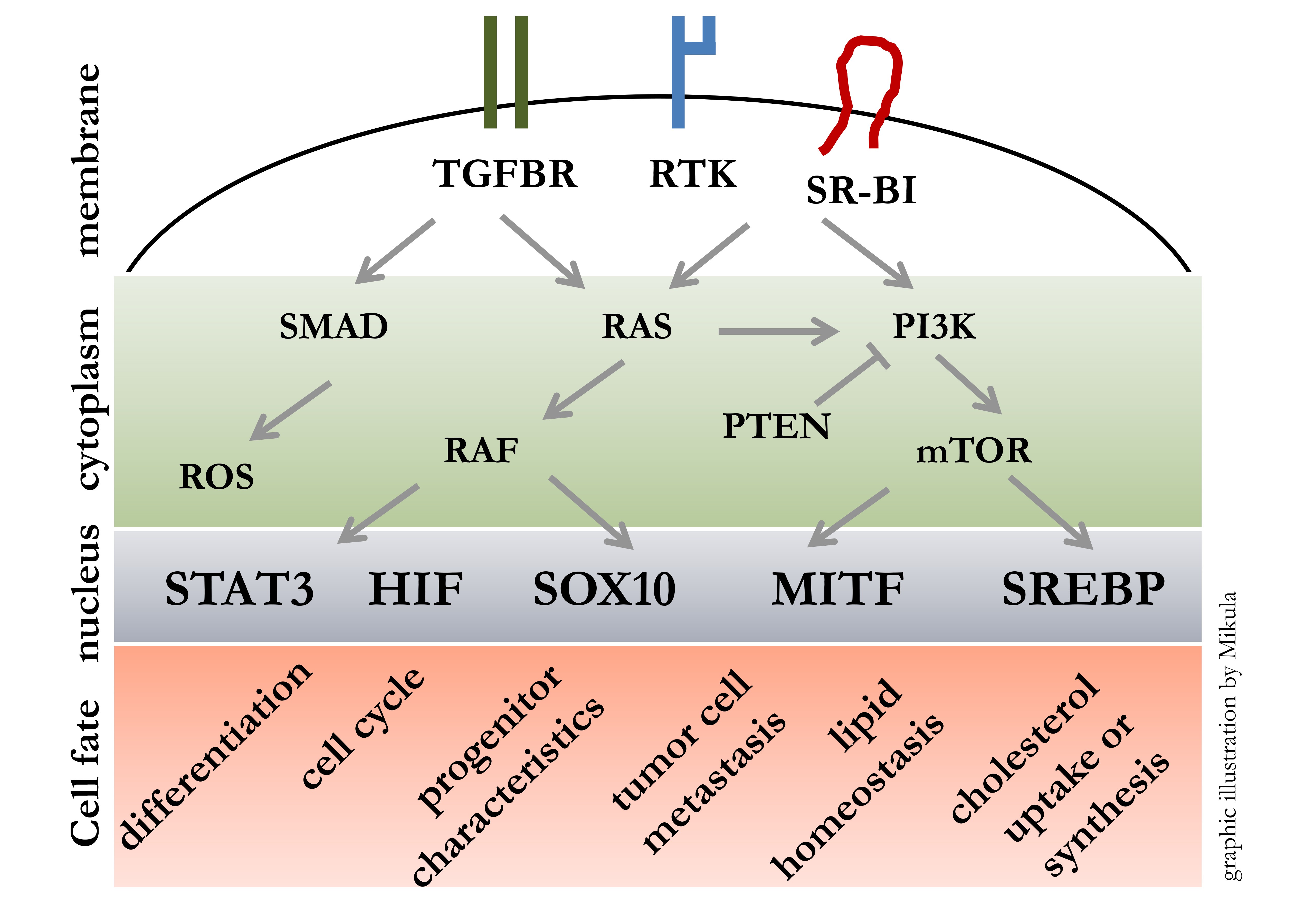
Malignant melanoma is a highly aggressive skin tumor with a strong tendency for metastasis and thus one of the most deadly cancers worldwide. Our goal is to transfer the knowledge generated from stem cell models [1-4] to cancer models in order to elucidate mechanism instrumental for disease progression. Therefore we study receptor driven cellular signalling pathways leading to activation of transcription factors with master regulatory roles. We are especially interested in outcomes affecting: cell cycle, cell migration, DNA damage, melanin generation, cholesterol uptake and synthesis.
Tumor progression is hallmarked by a dynamic interplay between highly proliferative versus highly migratory and invasive states. We have gained insights into these transitions by whole genome expression profiling, which enabled us to identify key regulating molecules like MET, SR-BI and STAT3 [5-7]. At best this mechanistic insights can lead to the development of novel biomarkers and furthermore it can lead to the development of novel therapeutic approaches [8].
Currently we further investigate the role of SR-BI (genename SCARB1) in melanoma. We found that SR-BI is necessary for metastatic melanoma cells to maintain the EMT phenotype, to perform glycosylation and to secrete VEGFA.
Applied Methods
We use human stem cells and human patient derived melanoma cells as in vitro and in vivo model systems.
Bioinformatics is used to perform whole genome association studies.
Confocal analysis of cells and tissue, RNA-seq, ATAC-seq and mass spectrometry are used as tools to characterise biologic outcome.
Links
References
- Rapamycin Maintains the Chondrocytic Phenotype and Interferes with Inflammatory Cytokine Induced Processes. De Luna Preitschopf, A.; Zwickl, H.; Nehrer, S.; Hengstschlager, M.; Mikula, M. Int J Mol Sci 2017, 18.
- Regeneration of injured tissue: stem cell dynamics at interplay with mTORC1. Mikula, M. Stem Cell Investig 2018, 5, 27.
- mTORC1 is essential for early steps during Schwann cell differentiation of amniotic fluid stem cells and regulates lipogenic gene expression. Preitschopf, A.; Li, K.; Schorghofer, D.; Kinslechner, K.; Schutz, B.; Thi Thanh Pham, H.; Rosner, M.; Joo, G.J.; Rohrl, C.; Weichhart, T., et al. PLoS One 2014, 9.
- Rapamycin-Induced Hypoxia Inducible Factor 2A Is Essential for Chondrogenic Differentiation of Amniotic Fluid Stem Cells. Preitschopf, A.; Schorghofer, D.; Kinslechner, K.; Schutz, B.; Zwickl, H.; Rosner, M.; Joo, J.G.; Nehrer, S.; Hengstschlager, M.; Mikula, M. Stem Cells Transl Med 2016, 5, 580-590.
- Malignant Phenotypes in Metastatic Melanoma are Governed by SR-BI and its Association with Glycosylation and STAT5 Activation. Kinslechner, K.; Schorghofer, D.; Schutz, B.; Vallianou, M.; Wingelhofer, B.; Mikulits, W.; Rohrl, C.; Hengstschlager, M.; Moriggl, R.; Stangl, H., et al. Mol Cancer Res 2018, 16, 135-146.
- MET expression in melanoma correlates with a lymphangiogenic phenotype. Swoboda, A.; Schanab, O.; Tauber, S.; Bilban, M.; Berger, W.; Petzelbauer, P.; Mikula, M. Hum Mol Genet 2012, 21, 3387-3396.
- STAT3 promotes melanoma metastasis by CEBP-induced repression of the MITF pigmentation pathway. Alexander Swoboda, Robert Soukup, Katharina Kinslechner, Bettina Wingelhofer, David Schoerghofer, Christina Sternberg, Ha Pham, Maria Vallianou, Jaqueline Horvath, Dagmar Stoiber, Lukas Kenner, Lionel Larue, Valeria Poli, Friedrich Beermann, Takashi Yokota, Stefan Kubicek, Thomas Krausgruber, Andre Rendeiro, Christoph Bock, Rainer Zenz, Boris Kovacic, Fritz Aberger, Markus Hengstschlaeger, Peter Petzelbauer, Mario Mikula, Richard Moriggl. BioRxiv. doi: doi.org/10.1101/422832
- Generation of metastatic melanoma specific antibodies by affinity purification. Schutz, B.; Koppensteiner, A.; Schorghofer, D.; Kinslechner, K.; Timelthaler, G.; Eferl, R.; Hengstschlager, M.; Missbichler, A.; Hundsberger, H.; Mikula, M. Sci Rep 2016, 6, 37253.
We have been or are funded by the following agencies:
Herzfeldersche Familienstiftung
Role of FGF signaling in Melanoma
Austrian National Bank
Reactivation of Embryonic Pathways in Melanoma
Austrian Science Fund FWF
FWF(P32979-B): Identification of a novel HIF regulation in human melanoma.
FWF (P 25336-B13): The Lipogenic Phenotype in Melanoma
FWF (P32979-B): Identification of a novel HIF regulation in human melanoma.
FWF (P 25336-B13): The Lipogenic Phenotype in Melanoma
Austrian Research Promotion Agency FFG
FFG (841291): Generation of a Metastasis Specific Antibody.