
Markus Hengstschläger, Univ. Prof. Mag. Dr.
Zentrumsleiter, GenetikerTel.: +43 (0)1 40160-56500
E-Mail: markus.hengstschlaeger@meduniwien.ac.at
Human stem cell biology
The array of established human stem cells contains cells of various sources, developmental stages and differentiation capacities. This diversity potentially creates new opportunities for stem cell research and therapy. It provides the rationale for the tailored use of stem cells to meet very specific demands while minimising risks, unwanted effects or other limitations associated with current approaches. For this concept to be valid however, the selection of one stem cell type over another needs to be based on the profound knowledge of its properties.
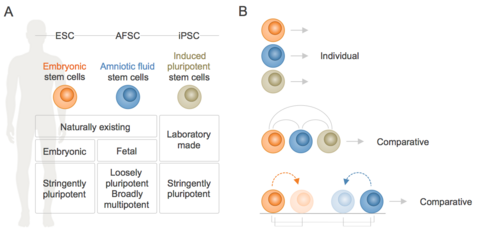
We are broadly interested in the identification and characterisation of stem cell traits, especially cell biological processes, which ultimately govern cell function. Our research is based on human stem cells of different origins including embryonic (ESC), amniotic fluid (AFSC) and induced pluripotent (iPSC) stem cells. Fetal AFSC, discovered in our lab in 2003, represent an intermediate between embryonic and adult stem cell states and share the ESC/iPSC property of trilineage differentiation but do not form tumours when injected into mice. Depending on the specific question, we study these cells individually, in direct comparison with each other or upon experimentally induced differentiation or self-organization into three-dimensional embryoids/organoids (Fig. 1). We also try to clarify whether AFSC are only mere bystanders of pregnancy or truly fulfill an in vivo biological function, specifically whether they contribute to pregnancy-associated fetal cell microchimerism. To study these questions, we use screening as well as hypothesis-driven approaches, a combination of cell biological, biochemical and computational technologies and a defined set of human stem cell lines.
For further reading see e.g.: Rosner, Hengstschläger: Amniotic fluid stem cells and fetal cell microchimerism. Trends in Molecular Medicine 19, 271; Kovacic, Rosner, Schlangen, Kramer, Hengstschläger: Distinct pathway interactomes control cell cycle, size and survival of human pluripotent stem cells. Scientific Reports 13, 9; Rosner, Schipany, Hengstschläger: Merging-high-quality biochemical fractionation with a refined flow cytometry approach to monitor nucleocytoplasmic protein expression throughout the unperturbed mammalian cell cycle. Nature Protocols 8, 602
Furthermore, our lab investigates how stem cells control their motility and the intertwined parameters of cell cycle, size and survival. We explore the molecular players and the signalling networks which govern these processes, and if or how these differ across stem cell types. In this context but not exclusively restricted to stem cell research, we have an already long lasting interest in the TSC/mTOR pathway (see e.g. Soucek, Yeung, Hengstschläger: Inactivation of cyclin-dependent kinase inhibitor p27 upon loss of the tuberous sclerosis complex gene-2. Proceedings of the National Academy of Science 95, 15653; Linke, Pham, Katholnig, Schnöller, Miller, Demel, Schütz, Rosner, Kovacic, Sukhbaatar, Niederreiter, Blüml, Kuess, Sexl, Müller, Mikula, Weckwerth, Haschemi, Susani, Hengstschläger, Gambello, Weichhart: Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nature Immunology 18, 293)
Another key aim is to understand how stem cells communicate with other cells ("the language of stem cells") and how this affects stem cell-related processes such as stem cell tumourigenesis. This includes the identification and characterisation of stem cell-derived paracrine stimuli (Figs. 2 and 3).
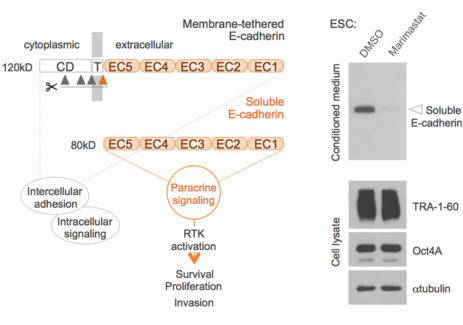
For further reading see: Rosner, Hengstschläger: Human pluripotent stem cells release oncogenic soluble E-cadherin. Stem Cells 34, 2443
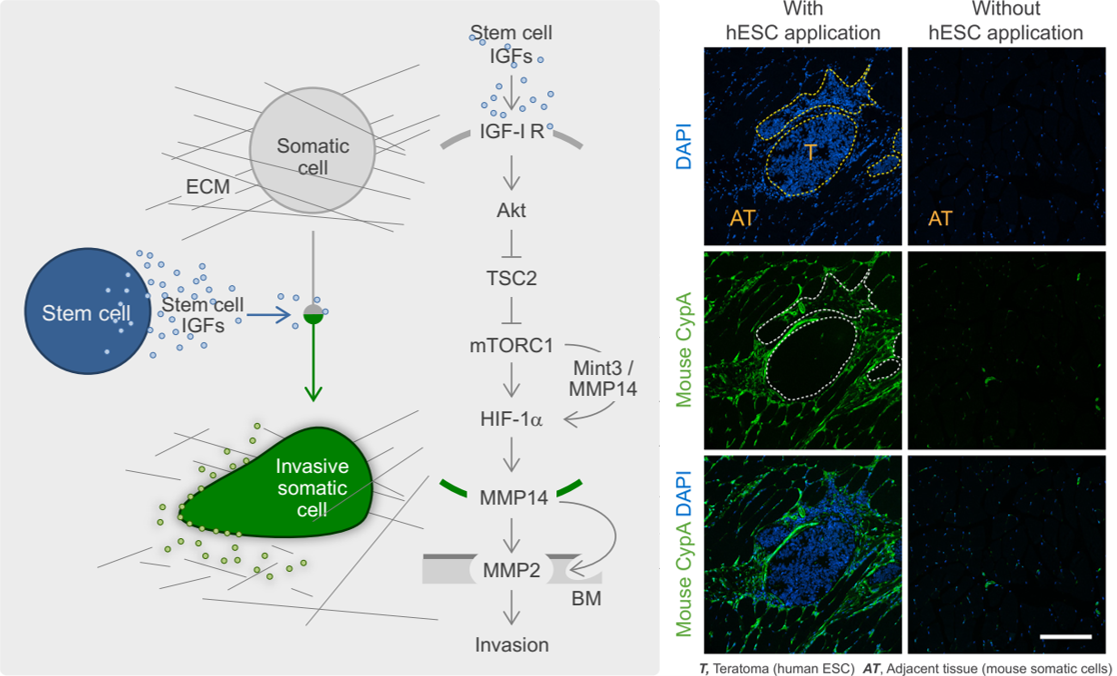
For further reading see: Rosner, Pham, Moriggl, Hengstschläger: Human pluripotent stem cells alter the invasive properties of somatic cells via paracrine activation of mTOR complex 1. Nature Communications 8, 595; Rosner, Hengstschläger: An antibody-based approach for the in situ evaluation of mouse contribution in human stem cell-derived xenografts. Nature Protocol Exchange doi: 10.1038/protex.2018.009
