
Raimund Oberle (Bauer), Mag. Dr.
PostdocTel.: +43 (0)1 40160-38022
E-Mail: raimund.bauer@meduniwien.ac.at
Lipid and lipoprotein metabolism and immunosuppression in cancer
Epidemiological data clearly demonstrate that patients suffering from obesity and the metabolic syndrome have a higher risk to develop cancer. For example, chronically elevated plasma insulin as well as leptin levels have been shown to favor tumor growth and progression, resulting in poor prognosis and higher mortality of obese cancer patients. Moreover, immunosuppressive myeloid cell populations accumulate in the tumor microenvironment (TME) of obese cancer patients, thereby fostering tumor growth and progression. By using syngeneic murine tumor models, biochemical and functional cell biological methods as well as immunological assays, we try to understand how metabolic perturbations in systemic lipid and lipoprotein metabolism affect the immunosuppressive behavior of myeloid cells in the TME. Specifically, we aim at the identification of novel obesity-related metabolic pathomechanisms that drive the activation and maturation of myeloid-derived suppressor cells (MDSC), thereby acquiring a highly immunosuppressive phenotype resulting in efficient inhibition of the anti-tumor T cell response.
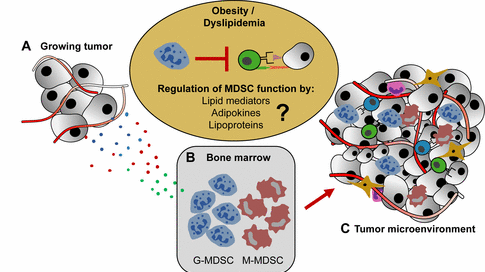
A, Growing, vascularized tumors secrete a plethora of soluble cytokines that communicate with B, the bone marrow niche, leading to the expansion of a heterogeneous population of immature, immunosuppressive myeloid cells (myeloid-derived suppressor cells; MDSC) of granulocytic (G-MDSC) and monocytic (M-MDSC) origin. C, MDSC then home into the TME, where they suppress anti-tumor T cell responses by various mechanisms such as arginine depletion and PD-L1 expression. Obese cancer patients and tumor mice frequently exhibit a profound degree of immunosuppression in the TME. However, the promoting forces that increase the immunosuppressive micromilieu in settings of obesity and dyslipidemia remain largely unknown.
- The HDL particle composition determines its anti-tumor activity in pancreatic cancer. Oberle R, Kührer K, Österreicher T, Weber F, Steinbauer S, Udonta F, Wroblewski M, Ben-Batalla I, Hassl I, Körbelin J, Unseld M, Jauhiainen M, Plochberger B, Röhrl C, Hengstschläger M, Loges S, Stangl H. Life Science Alliance, 2022 May 16. PMID: 35577388, DOI: 10.26508/lsa.202101317
- Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of anti-angiogenic therapy. Bauer R, Udonta F, Wroblewski M, Ben-Batalla I, Santos IM, Taverna F, Kuhlencord M, Gensch V, Päsler S, Vinckier S, Brandner JM, Pantel K, Bokemeyer C, Vogl T, Roth J, Carmeliet P, Loges S. Cancer Research 2018 PMID:29674477.
- BET-inhibition by JQ1 promotes proliferation and self-renewal capacity of hematopoietic stem cells. Wroblewski M, Scheller-Wendorff M, Udonta F, Bauer R, Schlichting J, Zhao L, Ben-Batalla I, Gensch V, Päsler S, Wu L, Wanior M, Taipaleenmäki H, Bolamperti S, Najafova Z, Pantel K, Bokemeyer C, Qi J, Hesse E, Knapp S, Johnsen S, Loges S. Haematologica 2018 PMID: 29567778.
- Mast cells decrease efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme B. Wroblewski M, Bauer R, Cubas Córdova M, Udonta F, Ben-Batalla I, Legler K, Hauser C, Egberts J, Janning M, Velthaus J, Schulze C, Pantel K, Bokemeyer C, Loges S. Nature Communications 2017 PMID: 28814715.
- A differentiation program induced by bone morphogenetic proteins 4 and 7 in endodermal epithelial cells provides the molecular basis for efficient nutrient transport by the chicken yolk sac. Bauer R, Tondl P, Schneider WJ. Developmental Dynamics 2019; PMID: 31691430.
- The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. Bauer R, Plieschnig JA, Finkes T, Riegler B, Hermann M, Schneider WJ. Journal of Biological Chemistry 2013 PMID: 23209291
